The safety profile of AMTAGVI® reflects the observed safety profile of the regimen, including lymphodepletion and IL-2 (aldesleukin)1
Adverse reactions in the C-144-01 study
Adverse Reactions Observed in at Least 10% of Patients Treated With AMTAGVI (N=156)1
| Adverse Reaction | All Grades n (%) | Grades ≥3 n (%) |
|---|---|---|
| Blood and lymphatic system disorders | ||
| Febrile neutropenia | 73 (46.8) | 73 (46.8) |
| Cardiac disorders | ||
| Tachycardiaa | 74 (47.4) | 12 (7.7) |
| Gastrointestinal disorders | ||
| Diarrhea | 73 (46.8) | 3 (1.9) |
| Vomiting | 68 (43.6) | 2 (1.3) |
| Nausea | 107 (68.6) | 4 (2.6) |
| General disorders and administration site conditions | ||
| Chills | 118 (75.6) | 8 (5.1) |
| Pyrexia | 95 (60.9) | 16 (10.3) |
| Fatigueb | 87 (55.8) | 8 (5.1) |
| Edemac | 66 (42.3) | 8 (5.1) |
| Investigations | ||
| Weight increased | 30 (19.2) | 2 (1.3) |
| Infections and Infestations | 42 (26.9) | 21 (13.5) |
| Infection with pathogen unspecifiedd1 | 30 (19.2) | 17 (10.9) |
| Infection with pathogen specifiedd2 | 19 (12.2) | 6 (3.8) |
| Adverse Reaction | All Grades n (%) | Grades ≥3 n (%) |
|---|---|---|
| Metabolism and nutrition disorders | ||
| Decreased appetite | 48 (30.8) | 2 (1.3) |
| Nervous system disorders | ||
| Headache | 33 (21.2) | 1 (0.6) |
| Encephalopathye | 27 (17.3) | 9 (5.8) |
| Renal and urinary disorders | ||
| Acute kidney injuryf | 31 (19.9) | 11 (7.1) |
| Hematuria | 22 (14.1) | 2 (1.3) |
| Respiratory, thoracic and mediastinal disorders | ||
| Hypoxiag | 37 (23.7) | 19 (12.2) |
| Dyspneah | 34 (21.8) | 13 (8.3) |
| Skin and subcutaneous tissue disorders | ||
| Rashi | 58 (37.2) | 15 (9.6) |
| Alopecia | 48 (30.8) | 0 (0) |
| Pruritus | 21 (13.5) | 0 (0) |
| Vascular disorders | ||
| Hypotensionj | 58 (37.2) | 17 (10.9) |
| Capillary leak syndrome | 21 (13.5) | 7 (4.5) |
| Hypertensionk | 21 (13.5) | 11 (7.1) |
Adverse reactions occurred from AMTAGVI infusion to 6 months (182 days) post infusion.
aTachycardia includes tachycardia and sinus tachycardia, atrial fibrillation, supraventricular tachycardia.1
bFatigue includes fatigue, asthenia, and malaise.1
cEdema includes edema, face edema, generalized edema, localized edema, edema peripheral, peripheral swelling, edema genital, scrotal edema, brain edema, catheter site edema, conjunctival edema, eyelid edema, laryngeal edema, macular edema, periorbital edema, pulmonary edema, vasogenic cerebral edema, and lymphoedema.1
d1Infection with unspecified pathogen includes cellulitis, conjunctivitis, cystitis, dermatitis infected, device related infection, diarrhea infectious, endocarditis, enterocolitis infectious, infection, meningitis, nasopharyngitis, neutropenic sepsis, pneumonia, pyuria, rash pustular, respiratory tract infection (RTI), rhinitis, sepsis, sinusitis, skin infection, urinary tract infection (UTI).1
d2Infection with mentioned pathogen includes bacteremia, candida infection, clostridium difficile colitis, cytomegalovirus infection or reactivation, Epstein-Barr virus infection, escherichia bacteraemia, fungal skin infection, herpes simplex, herpes zoster, metapneumovirus infection, oral herpes, oral candidiasis, pneumonia klebsiella, respiratory syncytial virus infection, skin candida, tuberculosis.1
eEncephalopathy includes encephalopathy, automatism, cognitive disorder, confusional state, depressed level of consciousness, disturbance in attention, hypersomnia, lethargy, leukoencephalopathy, memory impairment, mental status changes, paranoia, somnolence, and stupor.1
fAcute kidney injury includes acute kidney injury, anuria, azotemia, renal failure, renal tubular dysfunction, renal tubular necrosis, oliguria, and blood creatinine increased.1
gHypoxia includes hypoxia and oxygen saturation decreased.1
hDyspnea includes dyspnea, acute respiratory failure, orthopnea, respiratory distress, respiratory failure, and dyspnea exertional.1
iRash includes rash, rash generalized, rash maculo-papular, rash papular, rash pruritic, rash erythematous, and rash macular.1
jHypotension includes hypotension, blood pressure decreased, blood pressure systolic decreased, blood pressure diastolic decreased, and orthostatic hypotension.1
kHypertension includes hypertension, blood pressure increased, blood pressure systolic increased, and blood pressure diastolic increased.1
The majority of adverse events emerged within the first 15 days after treatment with AMTAGVI2
Treatment-emergent Adverse Events Over Time
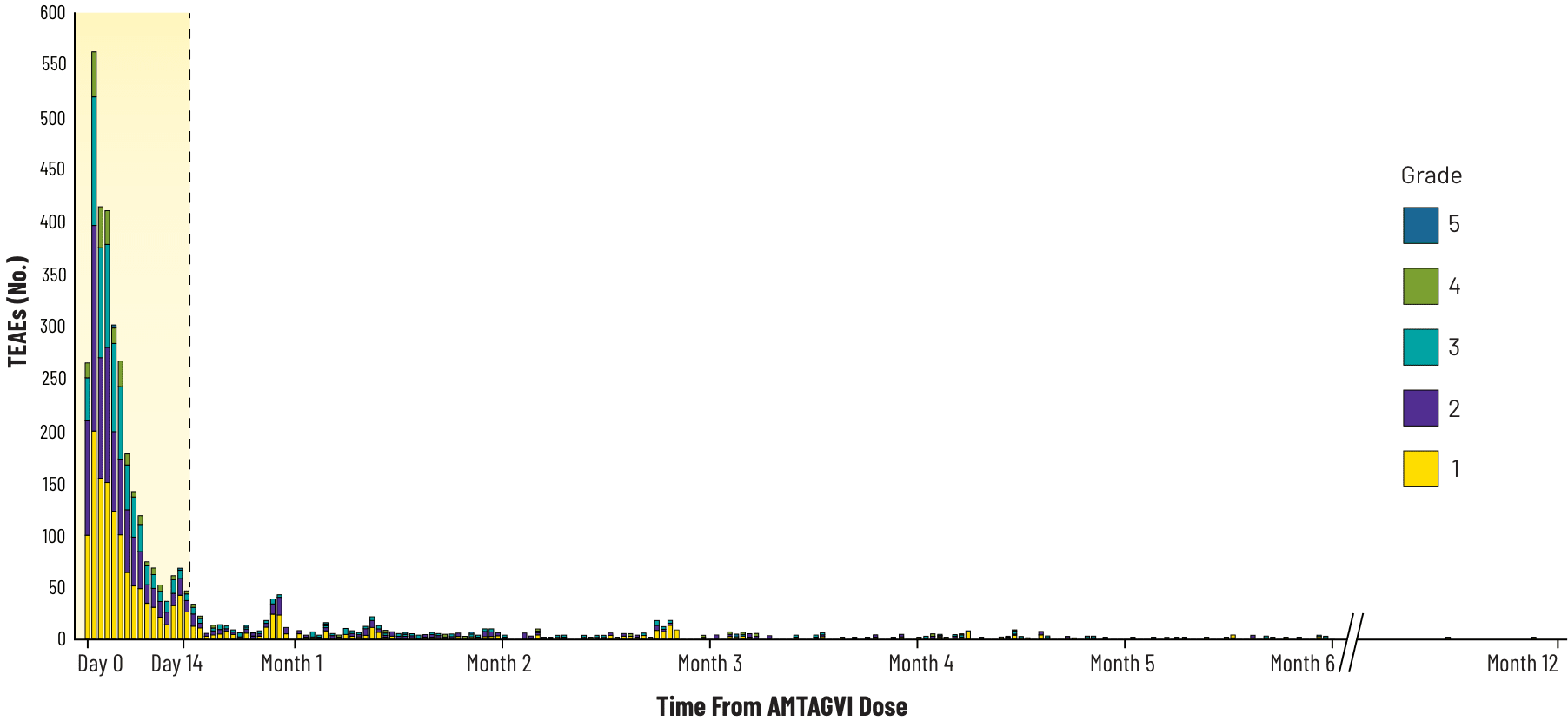
- Among 160 advanced melanoma patients who initiated the AMTAGVI regimen, there were 12 deaths (7.5%)1
- Grade 3 or higher cytopenia or pancytopenia which did not resolve to less than or equal to Grade 2 or lasted beyond 30 days post AMTAGVI infusion occurred in 45.5% of melanoma patients who received AMTAGVI. Filgrastim or a biosimilar product should be administered to patients beginning Day 1 after AMTAGVI and continuing daily until the absolute neutrophil count (ANC) is greater than 1000 per mm3 for 3 consecutive days, or per institutional standard.1
IL-2, interleukin-2; TEAE, treatment-emergent adverse event.